Abstract
Mantle cell lymphoma (MCL) is a rare and aggressive blood cancer comprising 5% of all non-Hodgkin lymphomas diagnosed annually. The median age of diagnosis is 68 years old, and while many patients initially respond to frontline treatment, relapse is common. To date, MCL remains incurable. There is an unmet need to develop novel therapeutic strategies for the treatment of patients with MCL.
Our group has identified protein arginine methyltransferase 5 (PRMT5) as a key driver of MCL pathogenesis. PRMT5 symmetrically dimethylates arginine residues on a number of proteins (p53, E2F1, p65) and histones (H4R3, H3R8, H2AR3), which support tumorigenesis. Selectively inhibiting PRMT5 has shown significant anti-tumor activity in preclinical MCL models, and a Phase 1 clinical trial with PRT543 (Prelude), a novel PRMT5 inhibitor, is underway.
While exploring pathways that converge with PRMT5 activity as potential avenues for combination treatment, we identified the intrinsic apoptotic pathway as an attractive target in MCL. BCL2 family proteins either promote or inhibit intrinsic apoptosis at the outer mitochondrial membrane through a dynamic set of binding interactions. Prior work has shown PRMT5 inhibition to drive the expression of multiple pro-death BCL2 family gene products (BAX, BAK, and BBC3[PUMA]) in MCL. We hypothesized that combining PRMT5 inhibition with BH3 mimetics, compounds that target pro-survival BCL2 proteins, would induce synergistic cell death in MCL.
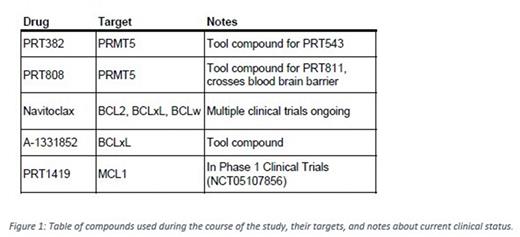
Selective PRMT5 inhibition with PRT382 inhibits the viability of MCL cell lines with an IC50 below 1uM in eight of nine lines (IC50 44.8nM - 1905.5nM). We chose six cell lines to test combination treatment ranging in sensitivity to BH3 mimetics and expression of BCL2 family proteins (CCMCL1, Z-138, UPN1, Granta 519, Mino, and Maver1). BH3 mimetics navitoclax (BCL2, BCLxL, and BCLw inhibitor), A852 (BCLxL inhibitor), and PRT1419 (MCL1 inhibitor) were found to have IC50s below 1uM in four, one, and three of the six cell lines respectively. Synergistic decreases in viability were tested via MTS assay and analyzed with the Loewe model of synergy. Mino, which showed sensitivity to all three mimetics, exhibited a synergistic reduction in viability with combination PRT382 treatment. Granta 519 and Z-138 exhibited a similar effect with the combination of PRT382 and navitoclax or A852. These observations were confirmed through BH3 profiling which measures functional apoptotic priming by quantifying mitochondrial depolarization after exposure to pro-apoptotic peptides. This assay supported MCL cell line variable dependence on BCL2, BCLxL, BCLw and MCL1, and increased sensitivity to BCL2 family protein targeting with PRMT5 inhibition. MCL tumor cells from patient derived xenograft models were tested ex vivo after treatment with 10mg/kg of the PRMT5 inhibitor PRT382 or vehicle. iBH3 flow-based analysis showed increased sensitivity to pan BCL2, BCLxL, and MCL1 inhibition.
Combinatorial treatment was tested in vivo using the PDX.AA.MCL model of ibrutinib resistant MCL. PRMT5 inhibition was achieved with PRT808 drugged chow (32.7 mg/kg) given five days on, two days off. Navitoclax (50mg/kg) and PRT1419 (30mg/kg) were dosed once weekly via oral gavage. Single agent PRT1419 provided no significant improvement over control mice as measured by circulating disease burden and survival. Navitoclax and PRT808 single agent as well as PRT808+PRT1419 regimens significantly slowed disease progression. PRT808+Nav showed the greatest reduction in disease progression and greatest survival advantage. Hematotoxicity was monitored via CBC and showed increasing thrombocytopenia as disease progressed. Of note, navitoclax dosing alone or in combination did not induce additional platelet loss.
Our results provide rationale for combining BH3 mimetics and PRMT5 inhibition in clinical trials as a novel treatment strategy for MCL.
Disclosures
Bhagwat:Prelude Therapeutics: Current Employment. Vaddi:Prelude Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Scherle:Prelude Therapeutics: Current Employment. Woyach:Janssen: Consultancy; Newave: Consultancy; Pharmacyclics: Consultancy; Schrodinger: Research Funding; Genentech: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Research Funding; Karyopharm Therapeutics: Research Funding; Loxo@Lilly: Research Funding; MorphoSys: Consultancy, Research Funding; ArQule: Consultancy. Elemento:Owkin: Consultancy, Current equity holder in publicly-traded company; One Three Biotech: Consultancy, Current equity holder in publicly-traded company; Volastra Therapeutics: Consultancy, Current equity holder in publicly-traded company, Research Funding; Janssen: Research Funding; Johnson and Johnson: Research Funding; AstraZeneca: Research Funding; Freenome: Consultancy, Other: Current equity holder in a privately-held company; Eli Lilly: Research Funding; Champions Oncology: Consultancy. Baiocchi:eLife (Journal): Other: Editorial board; CODIAK Biosciences: Research Funding; Atara Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Viracta Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal